European Union Horizon 2020 - Research and Innovation Framework Programme
- Project description
- Project objective
- Evaluation summary
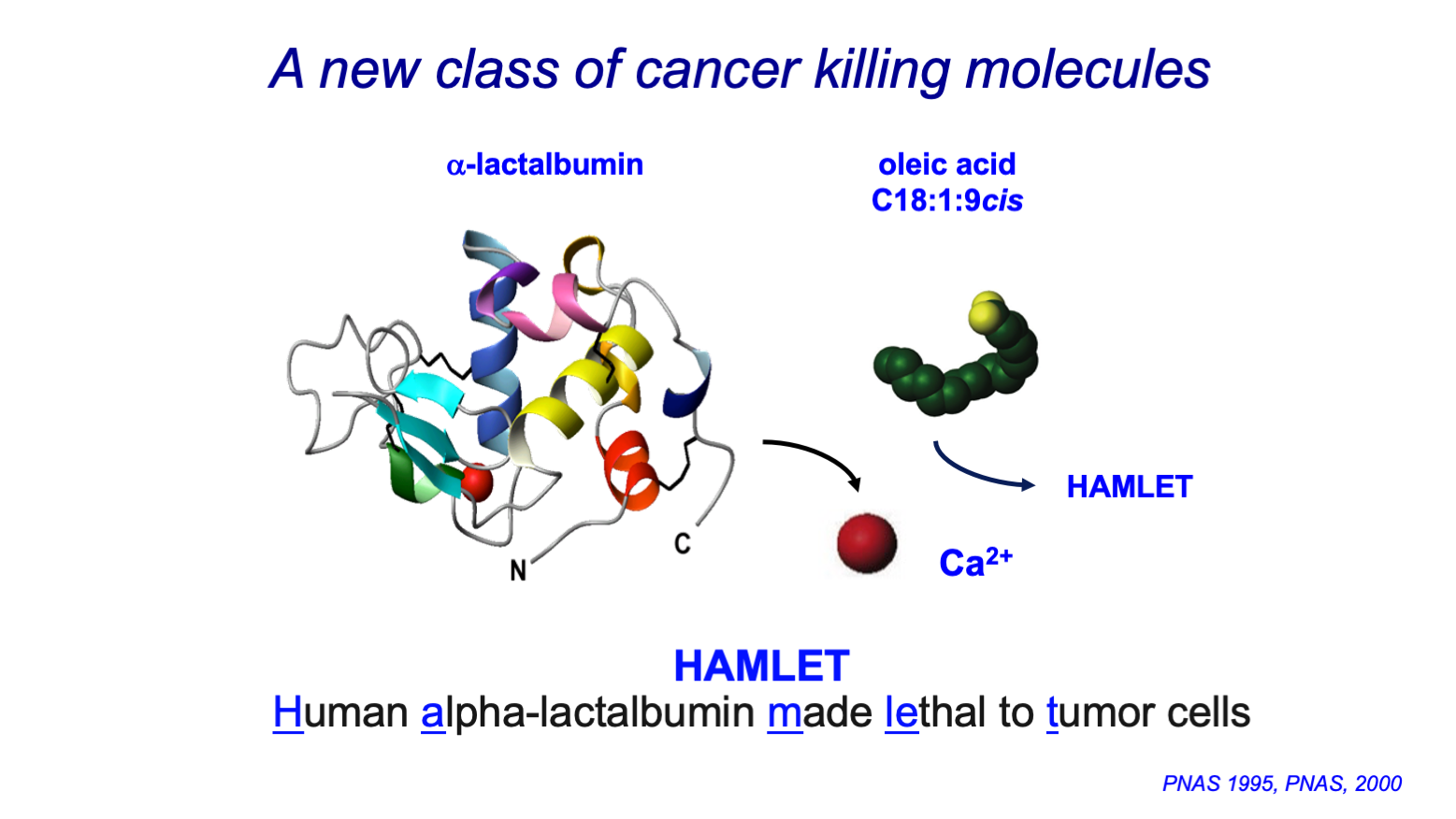
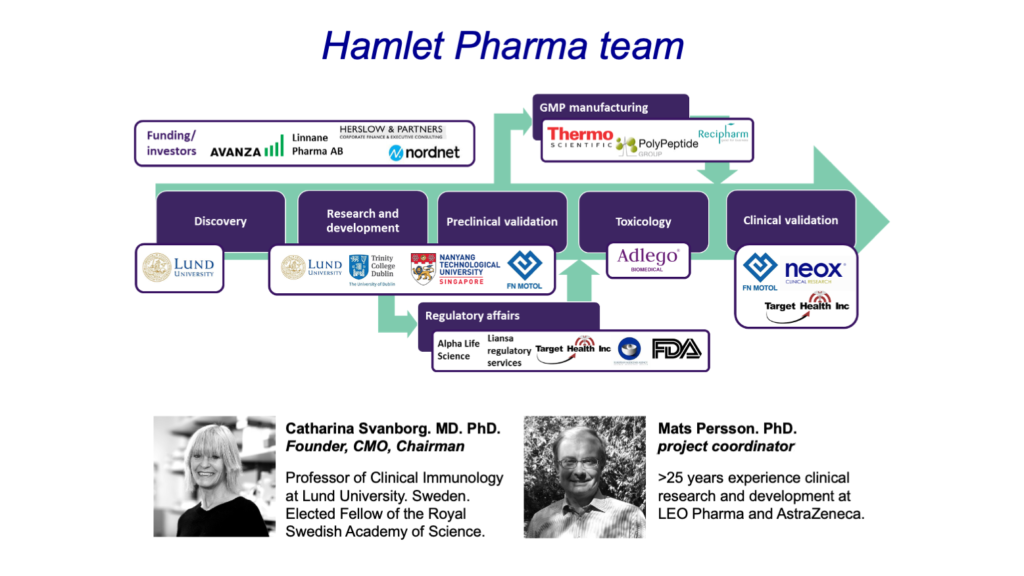
- The story of HAMLET
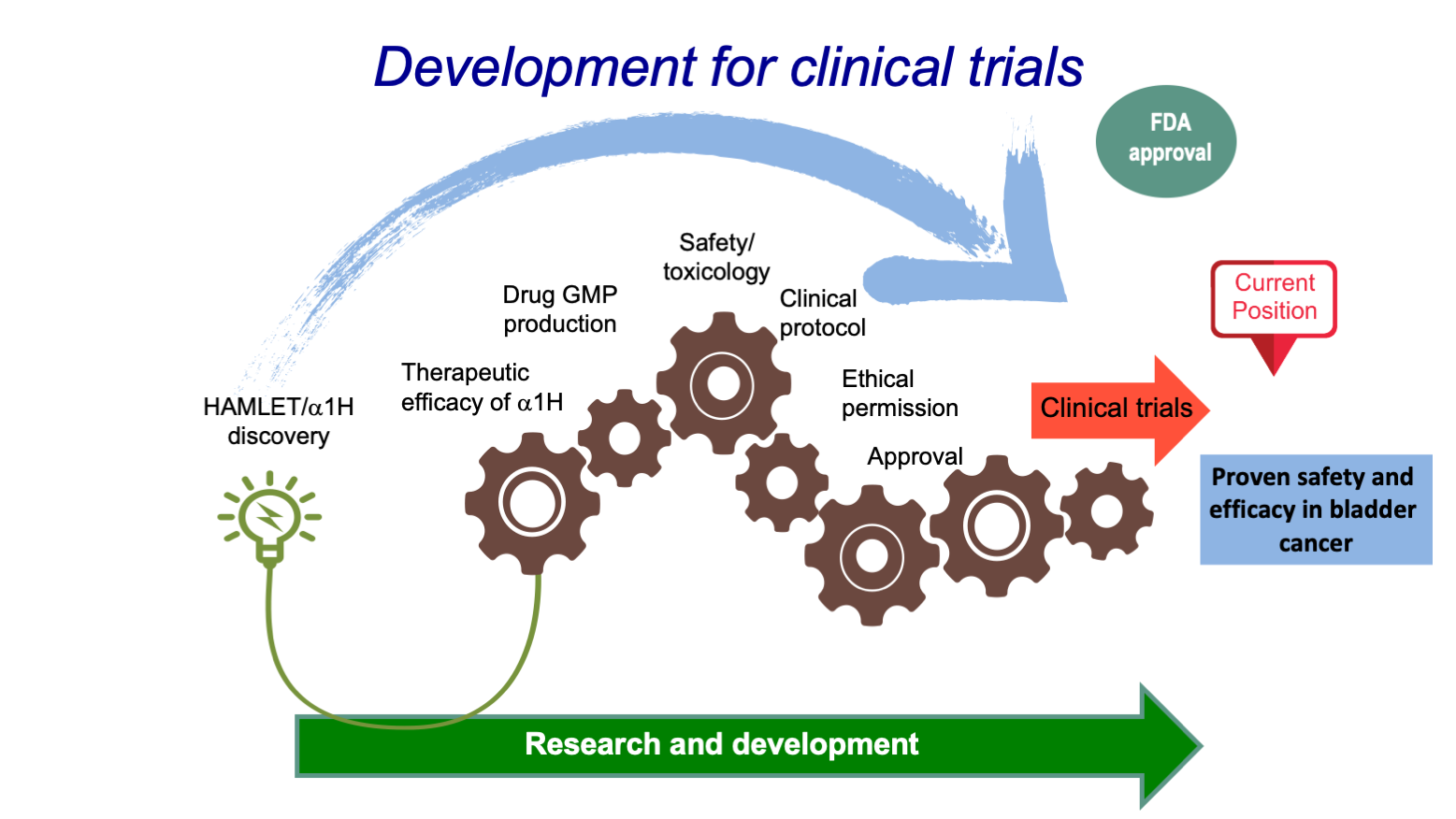
- Development for clinical trials
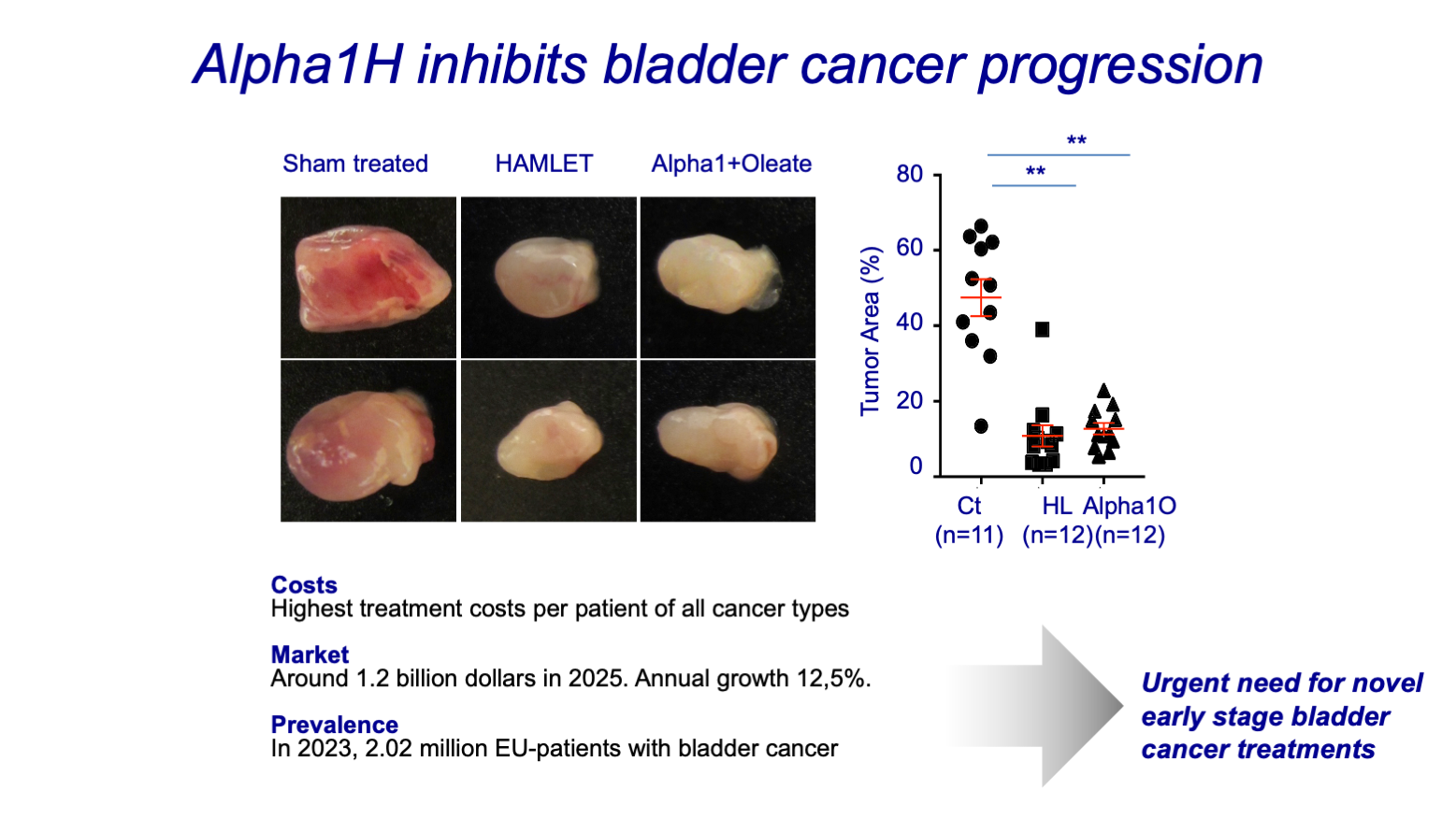
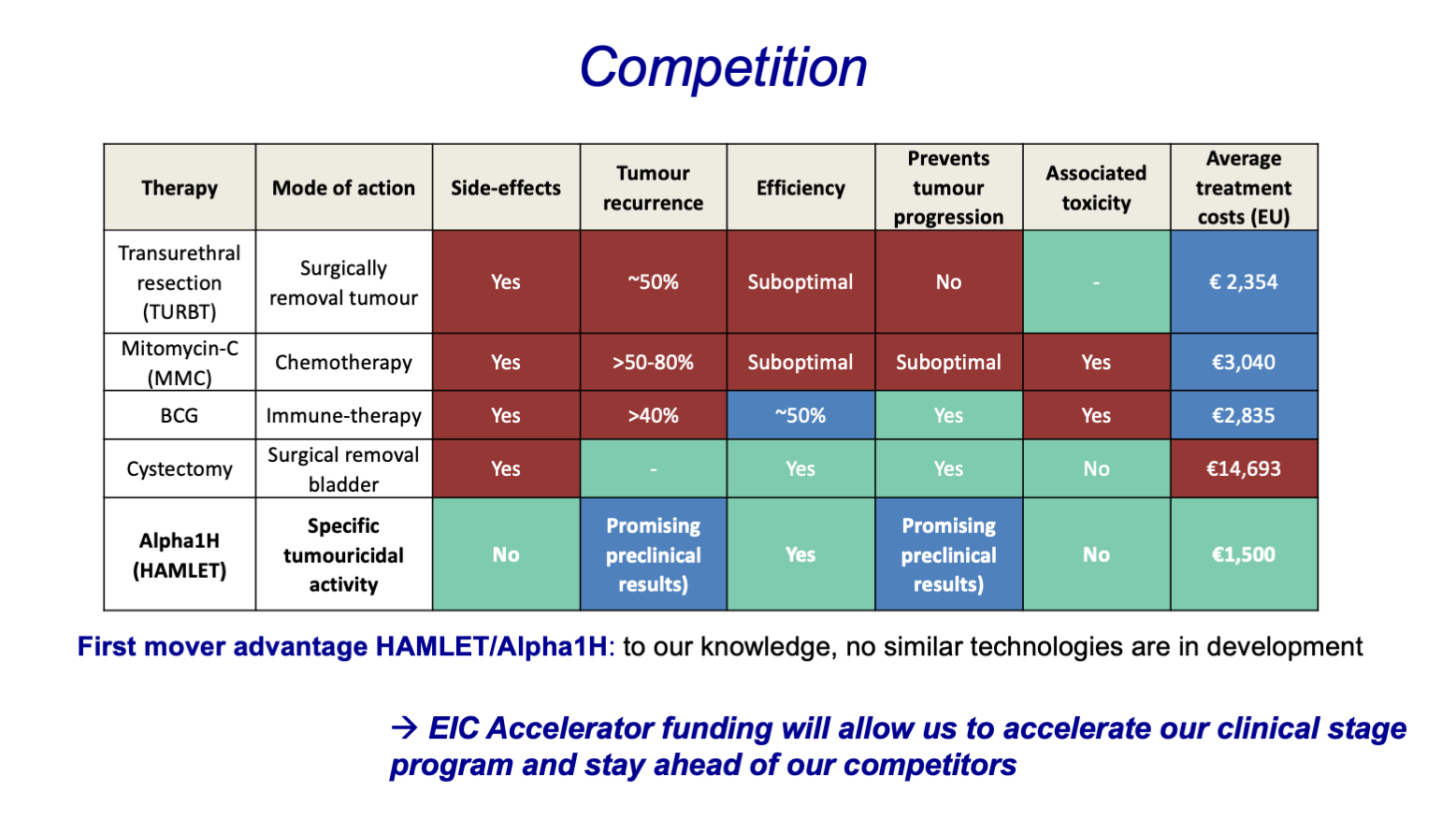
- Bladder cancer
- We have developed
- The funding will be used to
The ideal cancer treatment should combine great efficacy with high selectivity. The Swedish HAMLET Pharma AB develops revolutionary cancer treatments on a peptide-based platform for targeting and killing tumor cells with high precision. Their proprietary drug complex is derived from the human breast milk protein, alpha-lactalbumin, and represents a therapeutic solution with high tumor-killing capacity, high target specificity, no demonstrated toxicity, killing more than 40 different types of tumor cells. The EU-funded HAMLET-BC project will advance this drug complex in combination with the synthetic drug candidate Alpha1H into the clinical development pipeline as an early-stage treatment for bladder cancer, which has the highest recurrence rate among all cancer types.
The Swedish company Hamlet Pharma provides new cancer treatments based on a peptide-based molecular approach for targeting and killing tumour cells with greater precision. Its proprietary drug complex, HAMLET (‘Human Alpha-lactalbumin Made LEthal to Tumour cells’) is derived from the human breast milk protein, alpha-lactalbumin, and represents a ground-breaking therapeutic solution with high tumour-killing capacity, high target specificity, killing >40 different types of tumour cells and no demonstrated toxicity. As such, it has the potential to revolutionize cancer treatment.
Hamlet Pharma is now advancing HAMLET and the synthetic drug candidate Alpha1H along the clinical development pipeline as anti-cancer therapeutic for early stage bladder cancer. The company is focusing on bladder cancer given the high clinical need for more effective and safe treatment options. Bladder cancer has the highest recurrence rates (70%) and treatment costs per patient among all cancer types (total costs in Europe: >€4.9 billion). Recent successful outcomes include initial Phase I/II clinical trial data, proving that Alpha1H acts with high efficacy without any detectable toxicity.
With the EIC Accelerator funding, Hamlet Pharma will finalise Phase I/II clinical safety and efficacy studies and advance development of Alpha1H towards a Phase III trial (i.e. ready for licensing deal or IPO). Conversations with potential licensing partners and investors are ongoing. Alpha1H will be an important showcase of the impact HAMLET will have on cancer treatment (expected cumulative revenues: €350M five years after market approval). Generated revenues will be reinvested into development of HAMLET for other cancer indications.
- “The team is highly experienced with strong academic and business skills.”
- “The business model and commercialisation strategy appear feasible.”
- “The technology has the potential to be very disruptive, if proven. The market is ready to welcome a solution with limited side effects.”
- Impact – Score 4.75/5
- Excellence – Score 4.8/5
- Quality and efficiency of implementation – 4.7/5